Introduction
During invasive procedures, patients as well as the healthcare team are at risk for exposure to infectious agents through pathogen penetration of surgical gowns. Therefore, healthcare personnel should be aware of the relevant standards, guidelines, and professional recommendations that address surgical gowns. Through this knowledge, they are better equipped to make appropriate gown selections; thereby reducing exposure to blood, bodily fluids, and other potentially infectious material (OPIM).
Standards and Guidelines Related to Surgical Gowns
Several organizations have published standards and guidelines pertaining to the performance, classification, and use of surgical gowns. These organizations include the Food and Drug Administration (FDA), the American National Standards Institute (ANSI), the Association for the Advancement of Medical Instrumentation (AAMI), and ASTM International. Guidelines related to the use and selection of surgical gowns include those published by the Association of periOperative Registered Nurses (AORN). Those guidelines will be addressed in a later section.
Related Article
Testing Methods
There are three tests used for the ANSI/AAMI PB70 classification system. Two of the test methods are American Association of Textile Chemists (AATCC) tests. The AATCC 42 is the Spray Impact Penetration test. The AATCC 127 is the Hydrostatic Pressure test. The third test is the ASTM F1671 Penetration by Bloodborne Pathogens test. Given the results of the testing, a material may achieve an AAMI/AAMI PB70 classification rating of Level 1, Level 2, Level 3, or, the highest rating, Level 4. Further explanation of these tests follow.
- American Association of Textile Chemists and Colorists (AATCC) 42 Water Resistance: Impact Penetration Test.5 This test method is applicable to any textile fabric, which may or may not have a water-resistant or water-repellent finish. It measures the resistance of fabrics to the penetration of water by impact, and therefore can be used to predict the likely resistance of fabrics to water penetration. With this test, a volume of water is allowed to spray against a taut surface of a test material backed by a weighed blotter. The blotter is then reweighed to determine water penetration and the material is classified accordingly. The amount of water that penetrates the test fabric and soaks into the blotter on the other side is weighed in grams. The lower the weight of the blotter, the higher resistance demonstrated by the material.
- AATCC 127 Water Resistance: Hydrostatic Pressure Test.6 This test method measures the resistance of a fabric to the penetration of water under hydrostatic pressure. It can be used on all types of fabrics, including those that have been treated with a water-resistant or water-repellent finish. The amount of pressure required to force the water through the fabric is measured in centimeters of water pressure (i.e., cm); the higher the force in cm, the better the resistance.
- ASTM F1671/F1671M-13: Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Blood-Borne Pathogens Using Phi-X174 Bacteriophage Penetration as a Test System.7 This test method is used to measure the resistance of materials used in protective clothing to penetration by bloodborne pathogens using a surrogate microbe under conditions of continuous liquid contact. This test method has been specifically defined for modeling the viral penetration of HBV, HBC, and HIV transmitted in blood and other potentially infectious body fluids. Pass/fail scores are made based on the detection of viral penetration.
Not all Surgical Gowns are Created Equal
There are differences in surgical gown classification levels, construction, comfort and fit.
ANSI/AAMI PB70 Classification Levels
As just reviewed, there are four ANSI/AAMI PB70 classification levels for surgical gowns.4 An explanation of the testing results required for each of these levels follows.
Level 1. Materials assigned to this level are considered to be in a minimum protection classification and must undergo the AATCC 42 impact penetration test.5 To achieve level 1, all critical zone components shall have a blotter weight gain of no more than 4.5 grams with an acceptable quality level (AQL) of 4%. In other words, no more than 4% of the materials tested will fail.
Level 2. Assignment to this level requires that the material tested undergo the AATCC 42 impact penetration test as well as the AATCC 127 hydrostatic pressure test.6 To achieve level 2 classification, all critical zone components shall have a blotter weight gain of no more than 1.0 gram and a hydrostatic resistance of at least 20 cm with an AQL of 4%.
Level 3. Materials assigned to this level are required to undergo both AATCC 42 and AATCC 127 testing.5,6 To achieve level 3 classification, all critical zone components shall have a blotter weight gain of no more than 1.0 gram and a hydrostatic resistance of at least 50 cm with an AQL of 4%.
Level 4. Surgical gowns or other protective apparel achieve a Level 4 classification if they pass the ASTM F1671 test,7 which measures resistance to bacteriophage Phi-X174.
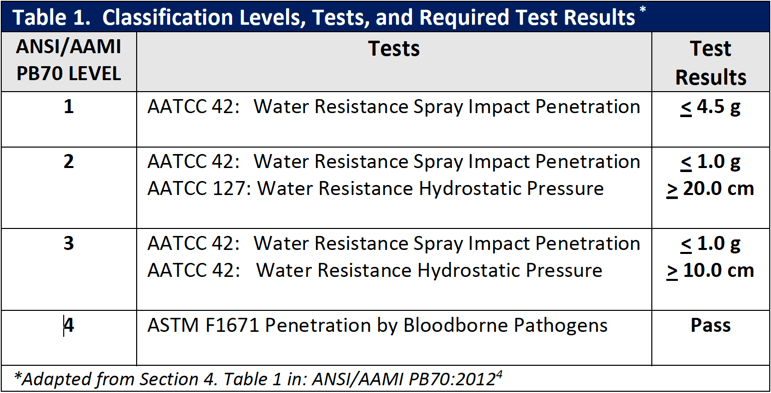
Construction
There are four basic types of surgical gown construction. As illustrated in Figure 1, they are non-reinforced gowns with non-reinforced fabric throughout; fabric reinforced gowns with additional layers of fabric on the gown front and sleeves; poly-reinforced gowns where areas of film are applied inside the gown fabric; and
breathable film gowns where the fabric has a breathable inner film layer throughout. Additionally, surgical gowns are made of either disposable single-use, nonwoven materials or reusable materials. With both of these types of materials (i.e., single-use and reusable materials), design and performance characteristics vary; the variations stem from trade-offs in costs, comfort, and the amount of barrier protection provided.
Figure 1 – Basic Types of Surgical Gown Construction
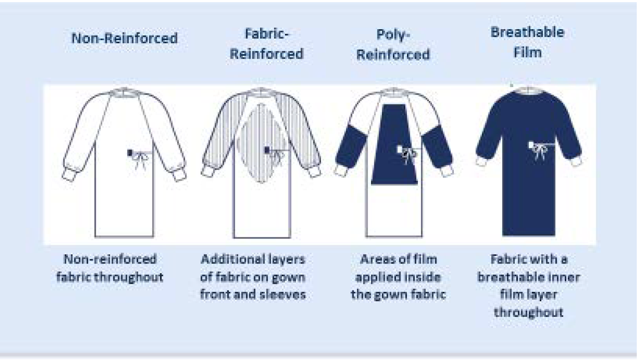
The ANSI/AAMI PB70:2012 Standard describes areas of a surgical gown.4 The entire front of the gown (see Figure 2 – areas A, B, and C) is required to have a barrier performance of at least level 1. The critical zone comprises at least areas A and B with the classification based on the lower-performing component. The back of the gown (area D) may be non-protective. The two general categories of protective materials are those with repellent construction and/or finishes and those with reinforcement by films. However, even within the same product, one area may be more resistant to liquid penetration than another.
Figure 2 – Example of surgical gown
Not all surgical gowns are alike and not all gown fabrics provide the same level of liquid barrier protection. Surgical gown designs vary in the level of protection provided for the body of the gown, the sleeves, and other areas. For fabric and poly-reinforced surgical gowns, reinforcements are only in critical zones, but in fluid intensive cases, where the surgeon is leaning on the patient, seated, or exposed to fluids and tissues, strikethrough may occur around the sides of the reinforced area. The ANSI/AAMI PB70 Liquid Barrier Standard classifies surgical gowns based on the critical zones. However, the width of critical zone A is not specifically defined. Depending on the type of procedure, there may be a risk for strikethrough at the junction of reinforcement and gown fabric. Because film patches are applied on the inside of poly-reinforced gowns, it may be difficult to see where the reinforcement is located.
Comfort and Fit
The fit of a surgical gown is fundamental to barrier protection and overall performance. As examples, a surgical gown that is too tight or too small will restrict movement or fail to adequately cover the wearer. If too big, the additional material may interfere with efficient movement. One source of discomfort related to surgical gowns is thermal sensation.8 That is, the wearer becomes too hot or too cold. Other issues that can negatively impact performance include personnel who are dealing with acute or chronic skin irritations and allergies attributed to the gown material. Gown materials that are stiff and unyielding can be uncomfortable to wear and impede efficient movement.
I am text block. Click edit button to change this text. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Strategies for Appropriate Selection of Surgical Gowns
Strategies for appropriate selection of surgical gowns include using professional guidelines and resources, such as those from the Association of periOperative Registered Nurses (AORN). Another strategy includes applying your knowledge of the ANSI/AAMI PB70:2012 Liquid Barrier Performance and Classification of Protective Apparel and Drapes Intended for Use in Health Care Facilities.
Association of periOperative Registered Nurses (AORN)
This professional association provides and regularly updates several recommendations related to the performance characteristics and comfort of surgical gowns that can be found in the AORN guidelines as summarized below.
Guideline for Product Selection9
This guideline provides recommendations for evaluating and purchasing medical devices and other products used for patient care including the following.
- A mechanism to select products should be developed; this includes establishing a multidisciplinary product evaluation and selection committee.
- The multidisciplinary committee should develop a process that guides product selection.
- Consistent requirements for each product being evaluated should be determined. Examples of product-specific requirements include: o compatibility with existing methods of disposal and reprocessing
- procedure-related requirements (e.g., resistance to penetration by blood and other body fluids)
- end-user preferences and requirements (e.g., for a surgical gown: comfort, the amount of protection from blood and body fluids, size, free from toxic ingredients or allergens)
- patient-related requirements (e.g., presence of infectious diseases)
- compliance with federal, state, local regulatory agencies, and with standard-setting bodies
- A product-specific evaluation tool should be developed with the use of unique, product-specific criteria, including: o safety, performance, quality
- efficiency, ease of use
- effect on quality patient care and clinical outcomes
- evidence-based efficacy
- analysis of the financial impact
- sterilization/reprocessing parameters (including degree of difficulty)
- environmental impact
- quality of the manufacturer’s instructions
Guidelines for Prevention of Transmissible Infections 10, 11
These guidelines provide guidance in implementing standard and transmission-based precautions (i.e., contact, droplet, and airborne precautions) to reduce the risk for infection. Recommendations related to surgical gowns include guidance from the Health Care Infection Control Practices Advisory Committee (i.e., the federal advisory committee that provides advice and guidance to the Centers for Disease Control and Prevention)12 and the Occupational Safety and Health Administration (OSHA). The OSHA mandate states that when health care workers are performing activities that generate splashes, sprays, aerosols of blood or OPIM, they must wear fluid-resistant gowns to protect them from exposure. 13
Guideline for Sterile Technique.14
This document states that surgical gowns that will be used in the practice setting should be evaluated and selected for safety, efficacy, and cost prior to purchase or use. Both the safety and efficacy of surgical gowns depend upon the design of the item as well as the materials from which they are constructed. Surgical gowns should be evaluated and selected for use based on several requirements that include the following.
Procedure-related requirements should be considered based on what is needed for the procedure where the products will be used (e.g., resistance to penetration by blood and other body fluids).
- End-user requirements should be considered including the amount of PPE needed based on preferences (e.g., fit, comfort) and anticipated risk of exposure to blood, body fluids, and OPIM.
- Environmental considerations should include the potential for reprocessing, recycling, waste reduction, resource conservation, and cost reduction that can occur without compromising the quality of patient care.
- Surgical gowns used during surgical and other invasive procedures must provide a barrier; they should also be resistant to tears, punctures, and abrasions.
- Tears, punctures, and abrasions may permit microorganisms, particulates, and fluids to pass between sterile and unsterile areas and thus expose patients and healthcare personnel to microbial contamination and bloodborne pathogens. Abrasions can negatively affect the barrier properties of the material by weakening it, thereby resulting in a tear or lint generation.
- The seams and points of attachment of surgical gowns should minimize the penetration of liquid and the passage of potential contaminants. Pressure or wicking on a seam or attachment point may result in liquid transfer between sterile and unsterile surfaces; therefore, one or both sides of the gown can become contaminated.
- • Surgical gowns should be non-abrasive as well as non-toxic in order to prevent tissue irritation, skin damage, or injury to personnel or patients.
- Barrier materials should be as lint free as possible. When lint particles are disseminated into the environment, bacteria attach to them; this bacteria-laden lint may settle into surgical sites and wounds and therefore may increase postoperative patient complications.
- Surgical gowns should be functional and flexible. Gowns that do not adequately perform and are unable to conform to and closely cover the wearer’s body may be difficult to use and may not provide protection from contamination by blood, body fluids, and OPIM.
- Surgical gowns should be selected for the procedure according to the barrier performance class of the product as stated on the label and the anticipated degree of exposure to blood, body fluids, and OPIM. Factors that should be considered when selecting surgical gowns include the:
- projected amount of blood loss
- volume of irrigation fluid used
- potential for spray, splash, pooling, or soaking
- length of the procedure
- potential for pressure or leaning
- type of procedure (e.g., open versus minimally invasive, superficial incision versus deep body cavity)
- team member’s role in the procedure
Healthcare personnel should select and use surgical gowns that are of appropriate size and sleeve length. A gown that is insufficient in size or sleeve length to cover the team member’s body may restrict movement, increase the potential for the scrubbed team member’s unsterile skin or apparel to come into contact with the sterile field, or fail to provide adequate coverage to prevent the personnel exposure to blood, body fluids, or OPIM. A gown that is of excessive size or sleeve length may brush against unsterile objects and surfaces. Surgical gowns should be large enough to sufficiently wrap around the wearer’s body and completely cover the back.
Surgical gowns should be selected so that the lower sleeves and gown cuffs:
- conform to the shape of the wearer’s arms
- are short enough to permit the gloves to fully cover the cuffs and mate properly with the lower sleeves
- are of adequate length to prevent gown cuffs from pulling out of gloves when the wearer’s arms are extended
ANSI/AAMI Standards Related to Surgical Gown Selection
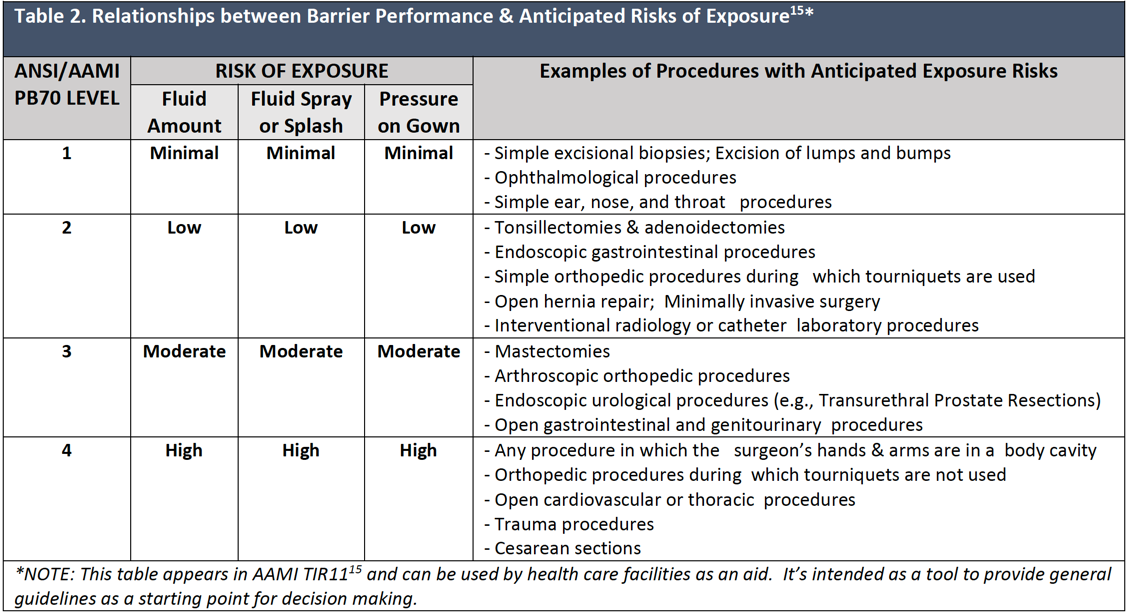
Surgical gowns should be selected for use according to the barrier quality of the item and the wearers’ anticipated exposure to blood and body fluids in accordance with OSHA guidelines for use of personal protective equipment.13 Surgical gowns are labeled by the manufacturer with the level of performance determined by the barrier properties of the area of the gown where direct contact with blood, body fluids, and other potentially infectious materials (OPIM) is most likely to occur.4 For example, short procedures during which there is little or no anticipated exposure to blood or body fluids can be completed successfully using a surgical gown with minimal barrier protection. As the complexity and length of the planned procedure increases, there may be increased potential for exposure to bloodborne pathogens, and it would be prudent to select a gown with greater barrier capability.
Table 2 gives examples of invasive procedures and the barrier performance classification levels based on the level of the anticipated blood and body fluid exposure. It is important to note that no two Level 4 gowns may be the same in terms of fit, performance, and comfort.
Summary
During invasive procedures, both the patient and members of the health care team are at risk for exposure to infectious agents through pathogen penetration of surgical gowns. Therefore, selection of surgical gowns with appropriate barrier protection for the task at hand is essential. This selection process is challenging as surgical gowns differ in barrier classification levels, construction, comfort and fit. Organizations that have published standards and guidelines pertaining to the performance, classification, selection, and use of surgical gowns include the FDA, ANSI, AAMI, ASTM International, and AORN. Health care personnel should be knowledgeable of information provided by these organizations and use this knowledge to enable them to make appropriate surgical gown selections.
Sources
-
Food and Drug Administration. August 1993. Premarket Notification [510 (k)] Submissions for Surgical Gowns and Surgical Drapes. Infection Control Devices Branch, Division of General and Restorative Devices.
Accessed June 3, 2017.2. Association for the Advancement of Medical Instrumentation news. (July 8, 2015). FDA proposes new criteria for surgical gowns. Accessed June 4, 2017.
3. Food and Drug Administration. December 9, 2015. Premarket Notification Requirements Concerning Gowns and Drapes Used in Health Care Settings; Guidance for Industry and Food and Drug Administration Staff. https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM452804.pdf. Accessed June 3, 2017.
4. American National Standards Institute, Association for the Advancement of Medical Instrumentation. August 2012. ANSI/AAMI PB70: Liquid Barrier Performance and Classification of Protective Apparel and Drapes Intended for Use in Health Care Facilities.
5. American Association of Textiles Chemists & Colorists. 2007. Water Resistance: Impact Penetration Test. AATCC 42-2007. Research Triangle Park, NC.
6. American Association of Textiles Chemists & Colorists. 2008. Water Resistance: Hydrostatic Pressure Test. AATCC 127-2008. Research Triangle Park, NC.
7. ASTM International. ASTM F1671/F1671M-13. 2013. Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Blood-Bourne Pathogens Using Phi-X174 Bacteriophage Penetration as a Test System. West Conshohocken, PA.
8. Zwolinska, M. and A. Bogdan. 2013. “Thermal sensations of surgeons during work in surgical gowns.” Int J Occup Saf Ergon 19(3): 443-453.
-
Association of periOperative Registered Nurses. 2017. Guideline for Product Selection. AORN Guidelines for Perioperative Practice, AORN, Inc.: 183-190.
-
Association of periOperative Registered Nurses. 2017. Ambulatory Supplement: Guideline for Prevention of Transmissible Infections. AORN Guidelines for Perioperative Practice, AORN, Inc.: 540-542.
-
Association of periOperative Registered Nurses. 2017. Guideline for Prevention of Transmissible Infections. AORN Guidelines for Perioperative Practice, AORN, Inc.: 507-539.
-
Siegel, J. D., E. Rhinehart, et al. 2007. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control 35(10 Suppl 2): S65-164.
-
OSHA. Occupational Safety and Health Standards, Toxic and Hazardous Substances, Bloodborne Pathogens, United States Department of Labor. 1910.1030. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=10051. Accessed June 3, 2017.
-
Association of periOperative Registered Nurses. 2017. Guideline for Sterile Technique. AORN Guidelines for Perioperative Practice, AORN, Inc.: 75-104.
-
Association for the Advancement of Medical Instrumentation. 2005. TIR11:2005: Selection and Use of Protective Apparel and Surgical Drapes in Health Care Facilities. Arlington, VA: 19-21.
